Non-Renal Systemic Lupus Erythematosus
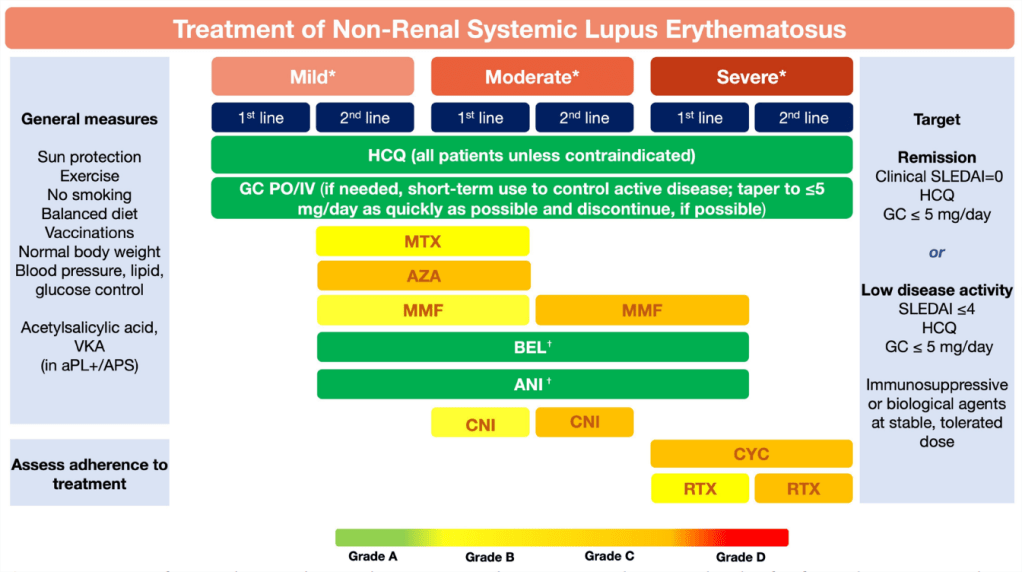
Figure 1 Treatment of non- renal systemic lupus erythematosus. Fanouriakis A, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis Epub doi:10.1136/ard-2023 224762
Top- to bottom sequence does not imply order of preference (eg, MTX, AZA and MMF are equal options for second- line therapy in mild disease or first- line therapy in moderate disease).
*Mild disease: constitutional symptoms; mild
arthritis; rash ≤9% body surface area; platelet count (PLTs) 50–100 × 109/L; SLEDAI≤6; BILAG C or ≤1 BILAG B manifestation.
*Moderate disease:moderate–severe arthritis (‘RA- like’; rash 9%–18% BSA; PLTs 20–50×109/L; serositis; SLEDAI 7–12; ≥2 BILAG B manifestations).
*Severe disease: major organ threatening disease (cerebritis, myelitis, pneumonitis, mesenteric vasculitis); thrombocytopenia with platelets<20×109/L; TTP- like disease or acute haemophagocytic syndrome; rash>18% BSA SLEDAI>12; ≥1 BILAG A manifestations.
†Recommendation of belimumab and anifrolumab as
first- line therapy in severe disease refers to cases of extrarenal SLE with non- major organ involvement, but extensive disease from skin, joints, and so on. The use of anifrolumab as add- on therapy in severe disease refers mainly to severe skin disease. For patients with severe neuropsychiatricdisease, anifrolumab and belimumab are not recommended.
ANI, anifrolumab; aPL, antiphospholipid antbodies; APS, antiphospholipid syndrome; AZA,
azathioprine; BEL, belimumab; BILAG, British Isles Lupus Assessment Group; CNI, calcineurin inhibitor; CYC, cyclophosphamide; GC, glucocortocoids;HCQ, hydroxychloroquine; IV, intravenous; MMF, mycophenolate mofetil; MTX, methotrexate; PO, per os; RTX, rituximab; SLEDAI, SLE Disease Activity
Index; VKA, vitamin K antagonists.
Fanouriakis A, Kostopoulou M, Andersen J, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis Epub doi:10.1136/ard-2023 224762
Lupus Nephritis

Figure 2 Treatment of lupus nephritis. Fanouriakis A, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. doi:10.1136/ard-2023 224762
Top- to- bottom sequence does not imply order of preference (similar to figure 1). #In addition to general
protective measures, as outlined in figure 1.
§BEL should always be given in combination with MMF or low- dose CYC as initial therapy, and with
MMF or AZA as maintenance therapy.
ˆCNIs should be given in combination with MMF. *Particularly recommended in the presence of poor prognostic factors: reduced eGFR, histological presence of cellular crescents or fibrinoid necrosis, or severe interstitial inflammation.
¶Extension of high- dose CYC to subsequent phase refers to severe LN cases, in which bimonthly or quarterly CYC pulses may be given following six monthly pulses.
†In relapsing/refractory disease, especially after failure to CYC- based regimens. ACEi, angiotensin- converting enzyme inhibitors; APS, antiphospholipid syndrome; ARB, angiotensin receptor blockers; AZA, azathioprine; BEL, belimumab; CNI, calcineurin inhibitor; CYC, cyclophosphamide; eGFR, estimated glomerular filtration rate; GC, glucocortocoids; HCQ, hydroxychloroquine; IV, intravenous; MMF, mycophenolate mofetil; MP, methylprednisolone; PO, per os; RTX, rituximab; SGLT2i, sodium glucose transporter 2 inhibitors; TAC, tacrolimus; Upr, urine protein; VKA, vitamin K antagonists; VOC, voclosporin.
